The simultaneous development of low-cost cameras and high energy synchrotron accelerators is enabling new methods to visualize plant structure and function. These methodological advances are ushering in a new era of visualizing plant anatomy and physiology for both research and teaching.
1. High resolution X-ray computed microtomography (microCT) enables 3D visualization of plants at micron resolution.
Because microCT imaging is non-invasive it does not have the sample preparation artifacts typical of other techniques. 3D images can be digitally sliced in any orientation, allowing unprecedented perspectives of plant anatomy. Furthermore, a full range of plant structures can be visualized, from high density, woody stems to low density and delicate flowers.
3D microCT images of Encelia (Asteraceae) leaves: (left) Encelia palmeri, (right) Encelia ventorum. The high density of leaf trichomes present on E. palmeri are difficult to quantify with other imaging techniques.
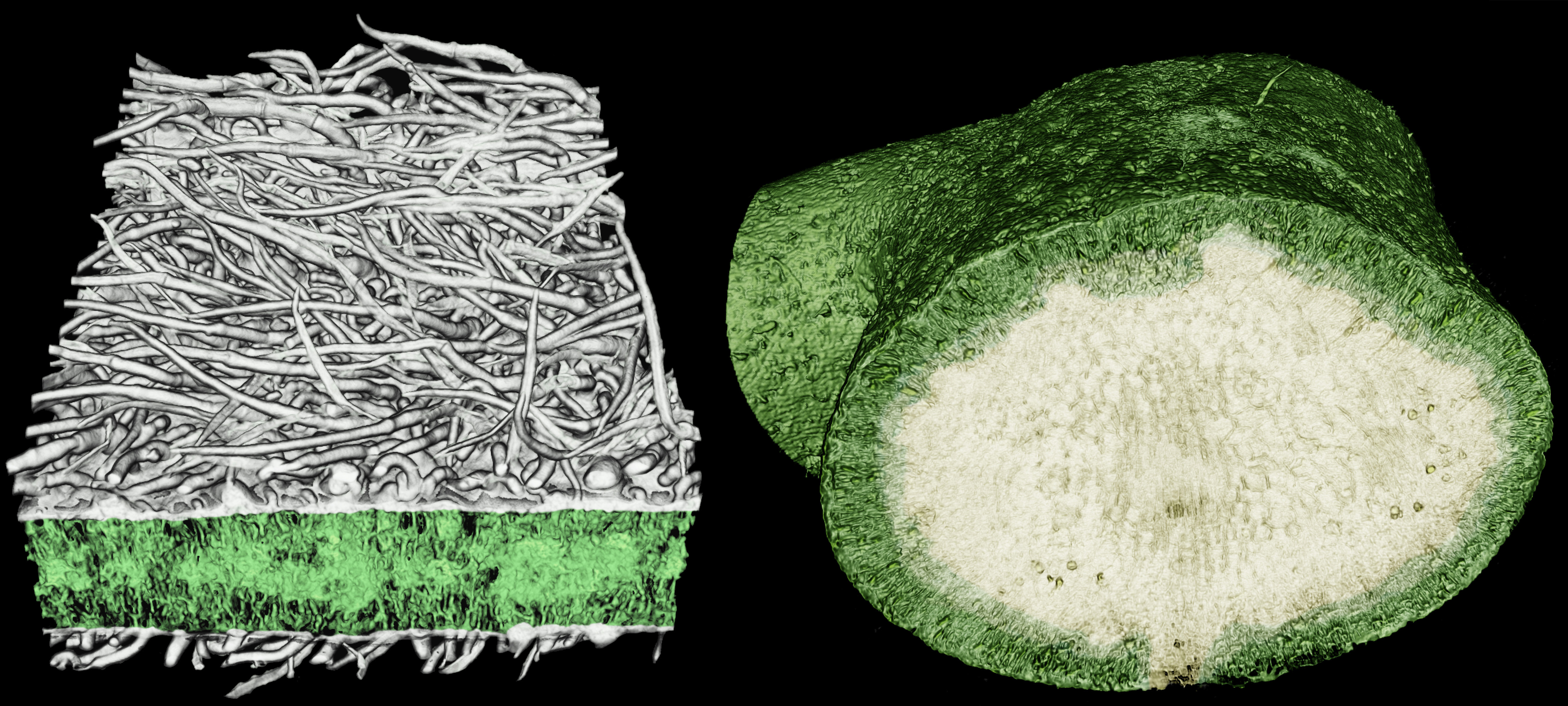
microCT imaging can also be used to distinguish between chemical constituents because compounds differ in the X-ray absorptivity. Below, crystals appear in the stem pith, distinct from other biological compounds (e.g. cellulose, lignin).
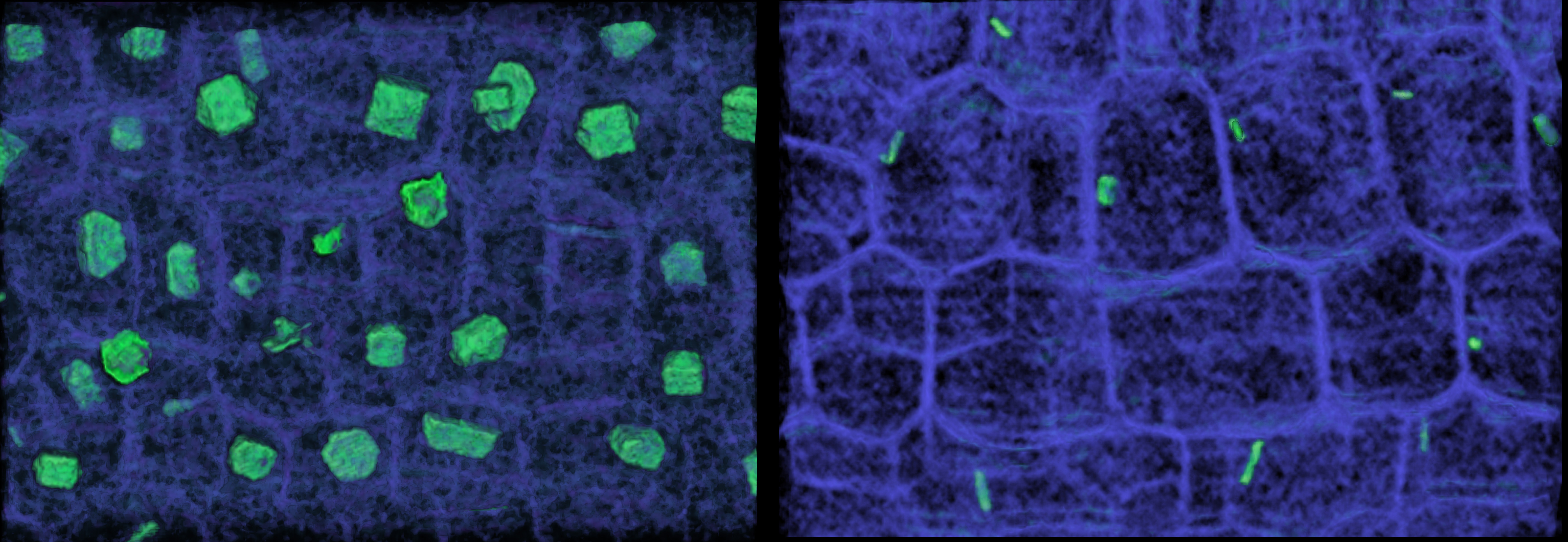
Visualizing the pith cells of stems reveals the localization of calcium oxalate crystals, using microCT imaging. (left) E. palmeri has a high abundance of large, faceted crystals in its stem pith. (right) E. ventorum has fewer, smaller, elongate crystals in its stem pith. Each image is 300 µm wide.
microCT imaging can also be used to visualize physiological processes in vivo. The plant vascular system is composed of a network of pipes that passively transport water throughout the entire plant (xylem) under negative tension. Under strong tensions, air can replace water in the vascular system, rendering it non-functional. The ability to withstand air embolisms varies widely among species and throughout the plant and has been a target of selection.
Vulnerability in the vascular system to air embolism-induced hydraulic failure can be visualized and quantified non-invasively.
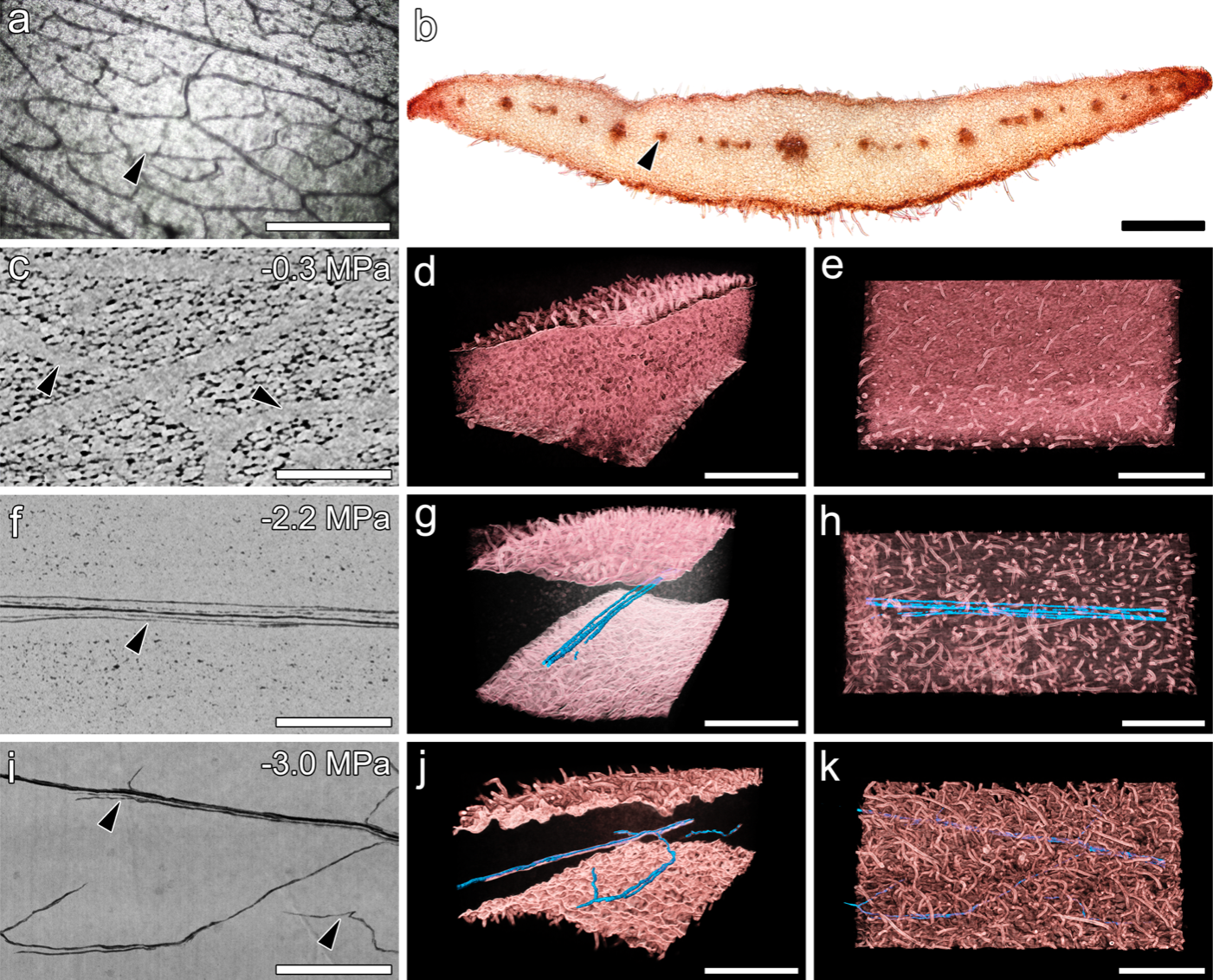
Vascular organization and embolism in Calycanthus floridus (Calycanthaceae) tepals. (a) Light microscopy showing veins in tepal. (b) Cross-section of a tepal showing vascular bundles in red. (c,f,j) 2D paradermal microCT images showing hydrated veins (light grey) and embolized vessels (dark grey in f,j). Colored images in d-k show tepal tissue in red with embolized vessels in blue.
2. 3D-printing enables visualizing plant physiology in vivo.
Visualizing stomatal dynamics can be tricky because they rapidly respond to environmental conditions. 3D-printing custom designed chambers that can interface with gas exchange systems and that can be positioned under a microscope enables complete control of the leaf microenvironment to visualize stomatal dynamics. Below, a Lilium leaf was induced to open and close its stomata by changing the humidity around the leaf.
3. Low-cost cameras enable parallelized visualization of embolism spread.
Because the refractive indices of water and air differ, embolism spread through the xylem network appears as a color change. Low-cost cameras can be deployed to track the timing and extent of embolism spread.
Below, time-lapse imaging of a desiccating Osmunda regalis (Osmundaceae) leaf reveals embolism formation and apread. Air embolisms appear as a darkening of the leaf veins in the left image, and they are simultaneously highlighted in color in the right image.
4. New imaging techniques can be used to teach plant anatomy interactively.
3D images can be annotated and viewed on a smartphone or 3D headset devices. Students can interactively explore these structures to learn plant anatomy in three dimensions.
Below is a 3D model of a Rhododendron petal that can be rotated and viewed either on the computer or immersively using a DIY virtual reality system such as a smartphone and Google Cardboard. The longest edge is less than 100 µm.
Related papers
Brodersen and Roddy. 2016. New frontiers in the three-dimensional visualization of plant structure and function American Journal of Botany
Roddy et al. 2018. Water relations of Calycanthus flowers: Hydraulic conductance, capacitance, and embolism resistance Plant, Cell & Environment
DiVittorio et al. In press. Natural selection maintains species despite widespread hybridization in the desert shrub Encelia Proceedings of the National Academy of Sciences